NEPTUNE: Neural plate targeting with in utero nano-injection
How does the intricately complex brain develop? And what is it that goes wrong in disorders such as cerebral palsy or pediatric brain tumors?
“If our brains were simple enough for us to understand them, we’d be so simple that we couldn’t.” (Ian Stewart).
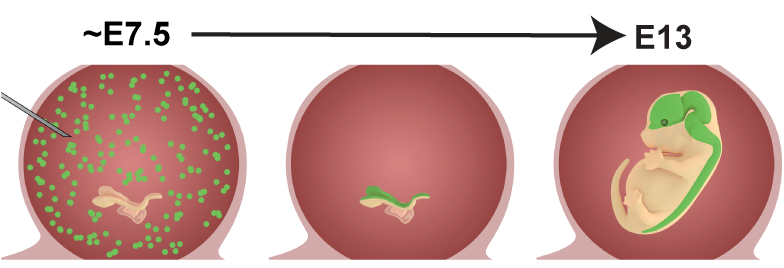
One of the driving forces in the lab is to try to understand how specific cells, structures, or systems develop in the brain. Dysregulation of brain development can result in developmental disorders with motor and/or cognitive defects, or brain cancer. In order to answer these questions, we have developed a high through-put in vivo approach to identify genetic components contributing to brain development, and determine the mechanisms by which these genes control development. We dubbed the technique NEPTUNE (neural plate targeting with in utero nano-injection). For more info see our preprint in bioRxiv, describing the method, or our collaborative publication in Nature Genetics in which we used NEPTUNE to lineage trace neural crest using barcode libraries.
miR-mRNA Structure-Function
What is the functional impact of different miR-mRNA structures?
In collaboration with Katja Petzold’s lab, we investigate the role of structure in determining the functional outcome of distinct miR-mRNA pairs. Using in vitro (cell culture) approaches and NEPTUNE, our lab aims to understand the in vivo role of miR-mRNA structure in determining functional outcomes.
Notch control of embryogenesis
How does abrogated Notch signaling result in Alagille syndrome?
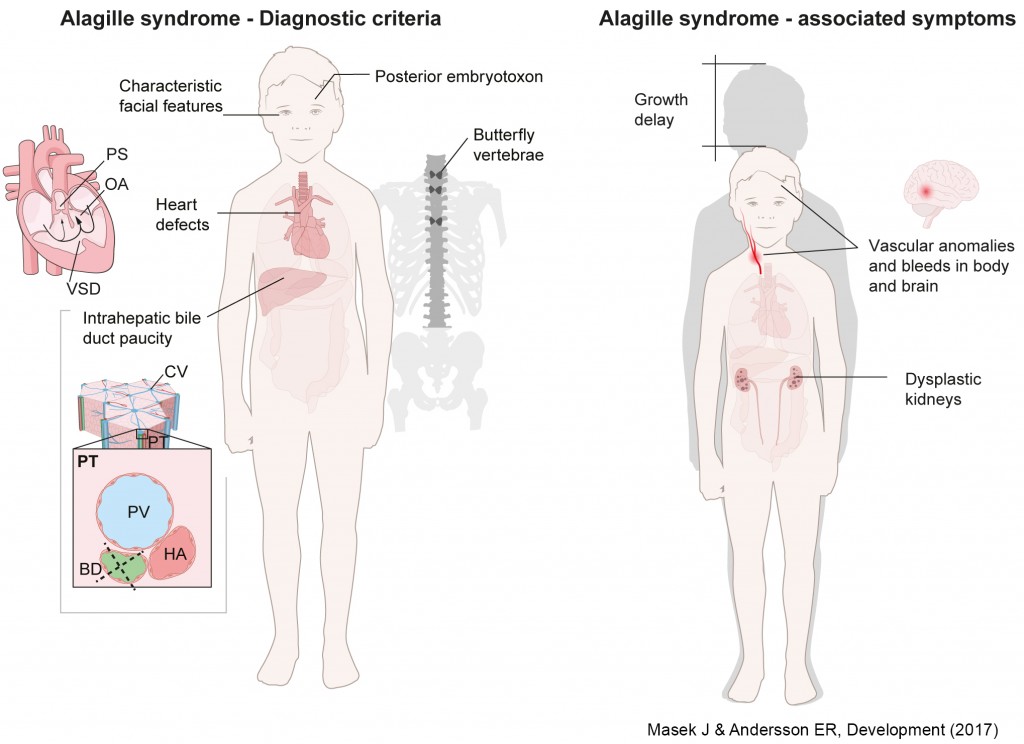
Alagille syndrome is diagnosed based on bile duct paucity, heart defects, characteristic facial features, butterfly vertebrae and posterior embryotoxon, followed by genetic confirmation of JAGGED1 (JAG1) or NOTCH2 mutations. However, other symptoms, including spontaneous vascular accidents and kidney dysfunction, pose significant problems for patients. Elucidating JAG1/NOTCH2-driven molecular and developmental processes will allow us to narrow down the best therapeutic approaches.
Bringing together epidemiological analyses, liver samples from patients, an Alagille mouse model, and high-throughput in vivo and in vitro assays, we aim to answer:
1. How does Notch regulate biliary tubule formation during development?
We developed a mouse model for Alagille syndrome (link) which revealed polarity defects in bile ducts rather than differentiation defects as a major cause of disease. We developed a technique to visualize, digitalize and analyze the biliary and vascular systems in 3D, which we nicknamed DUCT (for Double resin casting microCT). Using DUCT, we discovered that the biliary system in Alagille mice recovers via two architectural mechanisms: increased central (hilar) branching, and increased peripheral tortuosity. Together, these lead to reconstitution of a wild-type volume of biliary system. Our ongoing work is delving deeper into the cellular and molecular mechanisms regulating bile duct regeneration.
2. Why do patients with Alagille syndrome experience intracranial bleeds?
Chronic liver disease often results in coagulopathy and bleeding problems. However, patients with Alagille syndrome have been reported to suffer from bleeds in the absence of coagulopathy. Using the mouse model for Alagille syndrome that we developed, we found numerous vascular anomalies that could underpin spontaneous bleeding, including a vascular smooth muscle cell phenotype that mimics sparse vascular smooth muscle cells in CADASIL, a NOTCH3-related small vessel disease. We further investigated retinal fundus photographs from patients, and found that numerous vascular anomalies could be identified non-invasively. Read more in our pre-print.
3. How are Notch lateral inhibition and lateral induction orchestrated to fine-tune development of the organ of Corti?
The organ of Corti is a highly stereotypically patterned structure which senses and translates sound in our inner ear. Some patients with Alagille syndrome have been reported with sensorineural or conductive hearing loss. We are using the Alagille mouse model and NEPTUNE to investigate how Jagged1 contributes to hearing and how Notch components initiate and communicate to execute lateral induction and lateral inhibition.